Rules and regulations for drugs approved by Swissmedic and BAG
Reimbursement rules for drugs approved by Swissmedic and accepted for reimbursement by BAG are regulated by the SL List.
Forms for a cost-coverage confirmation for drugs with need for a preliminary approval can be found on the SGV page:
Kostengutsprachengesuche
Demandes préalables au médecin-conseil
Overview on the various reimbursement request statuses
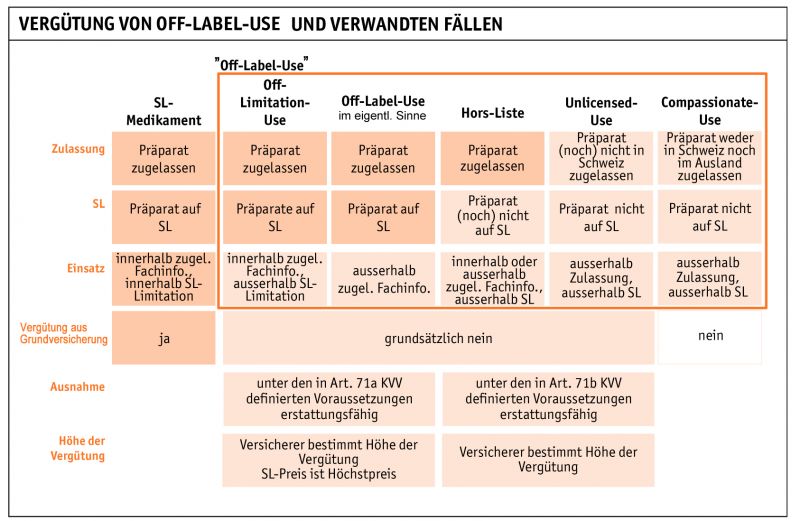
Off label Use
For cases not matching the official indication (off-label), the health insurance is allowed to ask a case-by-case evaluation and request the producing firm to contribute to the cost sharing. Art 71 applies here (read KVV text). The SGV has developed an algorithm, which should be applied in these cases.
Presentations to view:
Bachetto_Huber_2012
Seiler_2012
Proposed evaluation scheme for Art 71 a,b KVV cases by SGV: German, French
Off Limitatio Use
For cases outside of the official Limitatio fixed by BAG, health insurances apply the same rules as for Off-Label usage. We advise to seek direct contact with the Vertrauensarzt/Médecin-conseil.